– Validation Protocols and Reviews: Documentation of validation processes, including devices and method validations, which confirm that systems operate as supposed.
Any modifications to documents must be signed and dated, and the original information and facts really should continue being readable, with The rationale with the change recorded when essential.
Our group of gurus offers a complete spectrum of GxP consulting expert services, such as Excellent Documentation Methods, that can help corporations retain compliance, safeguard data integrity, and improve operational efficiency.
Portion 6 outlines that documents and records associated with the manufacture of Lively pharmaceutical components have to be prepared, reviewed, accredited, and controlled In keeping with composed treatments.
That is an open up-access report distributed underneath the conditions of your Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in almost any medium, supplied the original work is correctly cited.
In the course of the retention interval, originals or copies of records should be available in the establishment in which the things to do described in these documents transpired. Data that can be immediately retrieved from An additional place by electronic or other implies are satisfactory.
All staff members linked to documentation must be completely trained in GDP principles as well as importance of data integrity. Regular instruction sessions may also help reinforce these ideas and retain employees knowledgeable of regulatory updates.
The purpose of this work is always to present an introduction and normal overview on process validation of pharmaceutical producing system Primarily tablet manufacturing procedure with Exclusive reference to the necessities stipulated with the US Food stuff and Drug Administration (FDA). Quality is often an vital prerequisite whenever we consider any products. For that reason, prescription drugs need to be created to the very best high-quality levels.
For Price reduction: As a result of streamlined validation method, there’s a reduction in the amount of sampling and tests processes. This results in much less solution rejections and retesting, causing cost price savings.
Transient description of air flow systems. A lot more details need to be offered for critical areas here with opportunity chance of airborne contamination (schematic drawing of systems). Classification in the rooms utilized for the manufacture of sterile goods should be stated.
Entry should be restricted by passwords or other means and the result of entry of significant knowledge ought to be independently checked. Batch documents which might be electronically saved needs to be secured by back-up transfer on to magnetic tape, microfilm, paper, or other implies.
Some companies may also involve more skills or training in high quality administration systems or as per regulatory needs.
Write your title legibly in ink. Take into account that by signing information that you are certifying that the document is right and that you've got done the undertaking as per the defined course of action.
Step one of any circumstance will be to evaluate the software package Resource for its impact on health-related gadgets. If it'll have an effect on the “high get more info quality from the unit,” then validation is additionally necessary.
 Ben Savage Then & Now!
Ben Savage Then & Now! Jenna Jameson Then & Now!
Jenna Jameson Then & Now!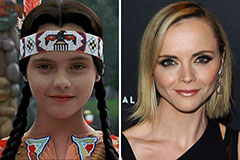 Christina Ricci Then & Now!
Christina Ricci Then & Now! Karyn Parsons Then & Now!
Karyn Parsons Then & Now! Naomi Grossman Then & Now!
Naomi Grossman Then & Now!